R/Pharma Summit
In-person event • Monday, September 15, 2025 • Atlanta, GA
Background
In 2018 and 2019, R in Pharma was in-person at Harvard University and focused on opportunities for direct interaction with speakers and guests.
These relationships and connections have grown into many exciting areas of Open Source drug development.
- In 2023 we partnered with Posit, hosting an in-person session at posit::conf(2023) in Chicago.
- In 2024, the session continued at posit::conf(2024) in Seattle.
- We’re thrilled to bring this back at posit::conf(2025) in Atlanta! 🎉
Over the last five years we’ve seen an explosive growth in the use of R and other open source technologies across drug development, with an increasing focus on pan-company collaboration.
The R/Pharma Roundtable Summit provides an open, collaborative, and inclusive environment to:
- Share learnings
- Understand common themes across our industry
- Establish collaboration opportunities
The focus is to foster in-person discussions about key items such as reproducibility and submissions, much like the original Harvard events.
🔗 Review the 2023–2024 roundtable summaries here:
rinpharma.github.io/roundtables
Call for Input
You can contribute to the discussion about the final agenda!
Join the GitHub discussions here:
👉 rinpharma-summit-2025 Discussions
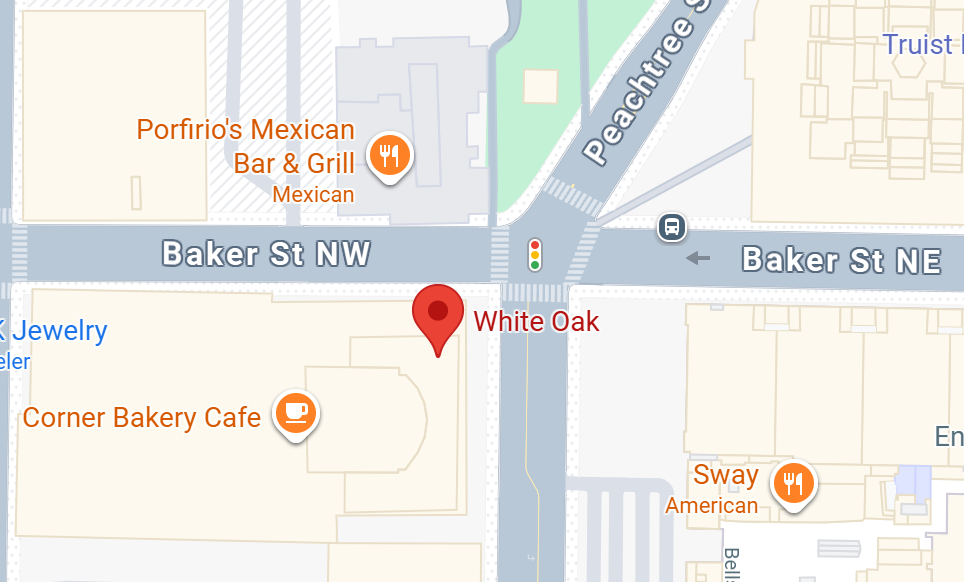
Location
Hyatt Regency Atlanta
265 Peachtree St NE
Atlanta, GA 30303, USA
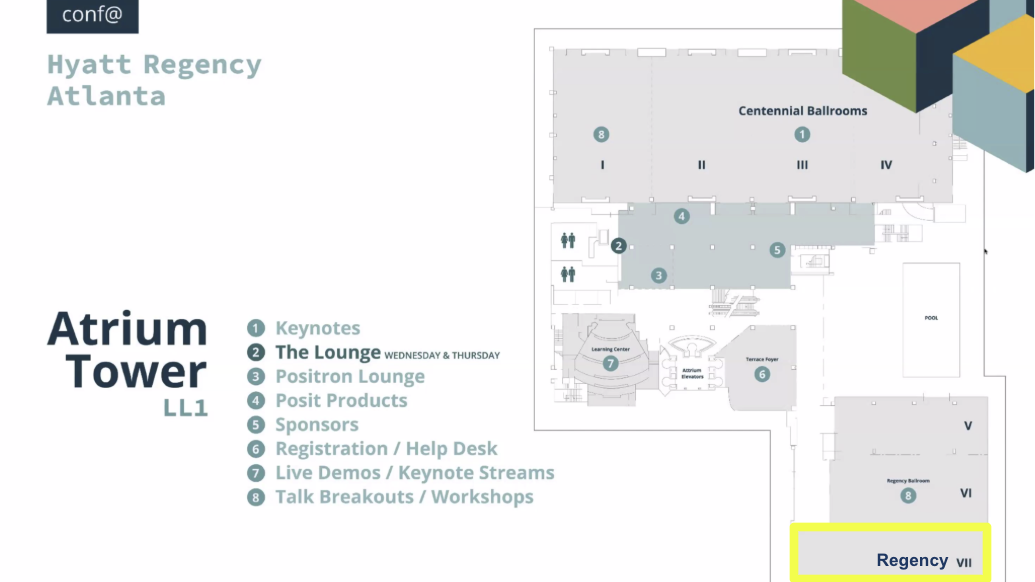
Meeting Room: Regency VII
Agenda
Roundtables
1. AI in Pharma
Chairs: Jeremy Wildfire, Sri Pavan Vemuri, Satish Murthy
- Best Practices for AI-Assisted R Coding
- What would an “AI-friendly” submission look like?
- Reimagine open-label trial deliverables with interactive display/AI
- Best Practices for AI Integration in Data Workflows
- Create more tables if needed for GenAI
2. Validation in 2025
Chairs: Olga Mierzwa-Sulima, Eric Nantz, Paulo Bargo
- Validating Open Source Tools with Stochastic Components
- Architectures to provision validated R packages
3. Python for Clinical Study Reports and Submission
Chairs: Yilong Zhang, Jonathan Tisack
- Medical Devices
4. SCEs in 2025
Chairs: David Thiriot, Phil Bowsher, James Black
- Open-source infrastructure to complement openstatsware and the pharmaverse
- Expanding the Pharmaverse beyond Clinical Reporting
5. Open-Source Change Management
Chairs: Ning Leng, Nate Mockler
- From Hacks to Habits — Tools & Tricks That Changed the Game
6. Implementation / Perspectives of CROs
Chairs: Douglas Henry, Steven Tan
- Expanding the Pharmaverse beyond Clinical Reporting
Audience
These round tables are for you if:
- You are an active contributor to the adoption of data science best practices across drug development
- You sponsor data science codebases
- You are an informatics professional designing and implementing GxP-conforming data science platforms
- You are interested in cross-pharma collaboration
Round Table Advisory Board
A mix of 50 people from various pharmaceutical companies, CRO and organization supporting the advancement of open source for drug development and clinical reporting.
Organising Committee
- Phil Bowsher — Posit
- James Black — Roche
- Harvey Lieberman — Novartis
- Katie Igartua — Tempus
This summit is run by Open Source in Pharma, a non-profit aiming to support the use of open source languages in Pharma.